The 6-in-1 vaccine combines Haemophilus influenzae type B and hepatitis B injections. In addition to preventing diphtheria, tetanus, pertussis, and polio, it can also prevent Haemophilus influenzae type B and hepatitis B, reduce the total number of injections by up to six , but also minimize the chance of side effects and discomfort.
Vaccine name |
Infanrix-hexaTM |
Infanrix-IPV-HIBTM |
Pharmaceutical company |
UK GSK | |
Suitable for |
Haemophilus influenzae type b infection needs to be prevented People who want to reduce the number of injections |
|
Effective Prevention |
Diphtheria, Tetanus, Pertussis, Polio, Haemophilus influenzae type B and Hepatitis B | |
Vaccination period |
It is recommended that babies start their first injection of the 6-in-1 vaccine when they are 6-8 weeks old After that, the second and third injections were given at 4 and 6 months respectively when the baby is 18 months old, a 5-in-1 vaccine booster injection will be given |
|
Side effect |
Loss of appetite, irritability, pain at the needle site, redness and swelling (less than 50 mm), mild fever and fatigue may occur after vaccination, but they will recover quickly | |
Not Suitable for |
Hypersensitivity to any component of the vaccine If encephalopathy of unknown etiology occurs within seven days of pertussis vaccination, pertussis vaccine should be discontinued immediately, but vaccination for other diseases should continue |
|
Precautions |
If the following situations occur after getting vaccinated with pertussis vaccine, medical staff should carefully consider whether to give pertussis-containing vaccine Fever over 40°C within 48 hours of vaccination, not caused by other reasons Symptoms of syncope or shock occur within 48 hours of inoculation Prolonged, persistent crying (more than three hours) within 48 hours of vaccination Having seizure within 3 days of vaccination (with or without fever)
**When Infanrix-hexaTM / Infanrix-IPV-HIBTM is in short supply, the company has the opportunity to replace it with a vaccine of the same quality - Hexaxim/Pentaxim (Sanofi, France). |
|

|
|
Vaccine name |
Hib Vaccine |
|
Suitable for |
Additional prophylaxis against Haemophilus influenzae type b infection is required |
|
Effective prevention |
Haemophilus influenzae type b causes meningitis and pneumonia. |
|
Inoculation time |
1st dose:2 months |
|
Common side effects |
After immunization, you may have loss of appetite, irritation, soreness at the injection site, redness and swelling (less than 50 mm), slight fever, and exhaustion, but these symptoms will pass rapidly. | |
Not suitable for |
Hypersensitivity to any vaccination component | |
Precautions |
You can alternatively select a 5-in-1/6-in-1 vaccine (containing Hib vaccine) to reduce the number of dose needed. | |
Although there is no effective treatment for hepatitis A, the effect of vaccination is very remarkable.
Vaccine Name |
Havrix 720 |
|
Pharmaceutical company |
UK GSK |
|
Suitable for |
Injection for children over 1 year old |
|
Effective Prevention |
The inactivated hepatitis A virus is the major component of the hepatitis A vaccination. After being injected into the human body, the vaccine stimulates the development of antibodies, resulting in protection to the hepatitis A virus. |
|
Vaccination period |
Two injections are required, separated by six months, and the validity term is approximately ten years. |
|
Side effect |
The most common side effects are moderate and include injection site soreness or swelling, headache, weariness, or lack of appetite. |
|
Not suitable for |
People with fever |
|
Precautions |
It is advised that children receive their first and second doses of hepatitis A vaccine at the ages of 1 and 1.5.
**When Vaqta is in short supply, the corporation has the option of substituting a vaccine of comparable quality - AVAXIM (Sanofi, France). |
|
肺炎球菌結合疫苗能夠有效地預防疫苗包含的血清類型的肺炎球菌所引致的嚴重侵入性肺炎球菌感染(如腦膜炎、菌血性肺炎及敗血病)。
5歲以下及50歲以上均為高危人士,故均建議接種,避免受肺炎球菌感染。
疫苗名稱 |
VAXNEUVANCE™ |
Prevenar20® 詳情 |
藥廠 |
美國 MSD |
美國 Pfizer |
適合對象 |
6週以上幼兒 |
|
清型數目 |
15種:1、3、4、5、6A、6B、7F、9V、14、18C、19A、19F、22F、23F、33F |
20種:1、3、4、5、6A、6B、7F、8、9V、10A、11A、12F、12B、14、18C、19A、19F、22F、23F、33F |
接種針數 |
||
特點 |
15價肺炎球菌疫苗有效針對血清3型肺炎球菌,免疫反應更強勁。 |
根據香港2023年統計,20價可針對約70%入侵性肺炎埔菌疾病個案 |
副作用 |
注射部位疼痛、腫脹、發紅; 疲勞、肌肉痛、頭痛、關節痛。這些副作用恉較輕微及只持續短時間。有輕微發燒、嘔吐或腹瀉等副作用,惟不適情況會很快消退。 |
|
不適宜注射 |
接受肺炎球菌結合疫苗後或對該疫苗的成份(包括抗原及蛋白載體)曾有嚴重的過敏反應 |
|
注意事項 |
可與滅活流感疫苗同時接種。 |
|
Currently, there is no medicine to treat rotavirus infection. We can only try to relieve symptoms, such as intravenous injection, to replenish lost water and electrolytes.
Even good hygiene is not very effective in preventing infection and the most effective way of preventing it is vaccination.
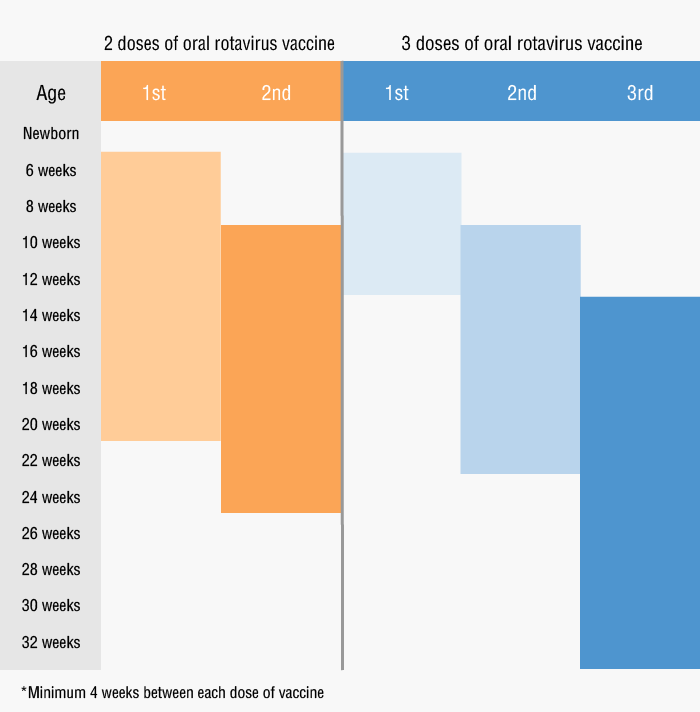
There are currently two oral rotavirus vaccines available in the market. Mothers can choose the most suitable product for their babies according to individual needs.
Vaccine name |
Rotarix |
RotaTeq (樂幼康) |
Doses |
2 doses |
3 doses |
Pharmaceutical company |
UK GSK |
US MSD |
Suitable for |
For babies from 6 to 24 weeks of age |
Suitable for babies from 6 to 32 weeks of age |
Effective prevention |
Protects young children from diarrhea caused by rotavirus infection |
|
Features |
replicate natural infection: generated from human rotavirus to replicate natural infection and give good protection |
Contains a variety of different serum types, covering 98% of the common types in Hong Kong |
Inoculation time |
It is recommended that young children take the first dose of vaccine at or before 20 weeks |
It is recommended that young children take the first dose of vaccine at or before 12 weeks |
Side effect |
Some babies may experience slight fever, vomiting, or diarrhea after receiving the rotavirus vaccine, but the discomfort will pass quickly. |
|
Not suitable for |
Hypersensitivity to any component of the vaccine |
|
Precautions |
Patients with vomiting symptoms should delay vaccination |
|
The Varicella vaccine injection provided in the government vaccine program and the 3-in-1 injection of MMR vaccine, a total of 3 injections throughout the injection process, and the newly launched MMRV vaccine, in addition to preventing chickenpox, also has measles, mumps and German measles, MMRV combines the varicella injection that needs to be injected separately and the 3-in-1 injection of measles, mumps and rubella. The combined vaccine not only reduces the number of injections by one, but also provides full protection to the infant before the 15th month. After two doses of vaccination, up to 99.8% of chickenpox immune responses can be elicited.
People who have been immunized against chickenpox can still get it (a condition known as "breakthrough infection"), but the symptoms are frequently milder or less characteristic, and the number of blisters is reduced. The rash is frequently maculopapular as opposed to vesicular. Those who have received vaccinations are shorter, however those who have not received vaccinations may have 300 or more irritating blisters at the time of commencement.
Vaccine name |
ProQuad |
|
Pharmaceutical company |
US MSD | |
Suitable for |
Intentionally reduce the number of injections |
|
Effective Prevention |
Chickenpox, measles, mumps, and rubella |
|
Inoculation time |
Two doses are required, and vaccinations are advised at 12 and 15 months (the time between the two doses should be at least one month). |
|
Side Effects |
After immunization, pain, slight fever, redness or rash at the injection site, and swollen glands in the cheeks or neck may occur, but recovery is quick. If these symptoms arise, they usually do so within two weeks following vaccination. After the second dose, it is less likely to occur. |
|
Not suitable for |
Hypersensitivity to any vaccination components |
|
Precautions |
If MMR or varicella vaccination was given at 12 months of age, it can be given between 15 months and 6 years of age. Inject the second dose of MMRV combination vaccination, allowing at least one month between injections. |
|
Age |
Current Government Vaccination Program |
Medtimes MMRV Vaccination Service |
|
1 year old |
Varicella vaccine (1st dose)
|
MMRV Vaccine (1st dose) |
|
15 months old (the ealiest) |
- |
MMRV Vaccine (2nd dose) |
|
18 months old |
MMR vaccine (3rd dose) |
- |
|
6 years old (Primary 1)
|
MMR vaccine (3rd dose) |
- |
|
|
Total 3 doses |
Total 2 doses |
預防腦膜炎雙球菌的方法,離不開增強抵抗力、經常保持雙手清潔、咳嗽或打噴嚏時應掩蓋口鼻、保持室內空氣流通等,但最有效的方法為疫苗接種。
預防腦膜炎雙球菌的方法,離不開增強抵抗力、經常保持雙手清潔、咳嗽或打噴嚏時應掩蓋口鼻、保持室內空氣流通等,但最有效的方法為疫苗接種。
香港現時已有疫苗可預防日本腦炎。
雖然日本腦炎在香港並不常見,但市民如果計劃於疾病傳播季節到高危地區旅遊、或到郊區並大部份時間進行戶外或夜間活動,可考慮於前往該等地區旅遊前接種預防疫苗。
疫苗名稱 |
Imojev
|
||
藥廠 |
法國 SUBSTIPHARM |
||
適合對象 |
準備前往日本腦炎流行的地區(特別是郊區)逗留一個月或以上的旅遊人士 | ||
有效預防 |
日本腦炎 |
||
接種時間 |
滿9個月 (9個月至17歲需接種兩針,兩針相隔12-24個月) |
||
副作用 |
主要是接種部位出現不同程度的紅腫和疼痛,也可能有發冷、頭痛及發燒等情況 |
||
不適宜注射 |
過去曾對日本腦炎疫苗有過敏反應 |
||
注意事項 |
需注射兩針,相隔1至2年 |
||
Vaccine Name |
Respiratory Syncytial Virus (RSV) Preventive Antibody Injection - Beyfortus |
|
Suitable for |
All infants under 1 year old |
|
Effective prevention |
Respiratory syncytial virus (RSV) | |
Vaccination time |
0-1 years old (weight <5kg 50mg 1 dose; ≥5kg 100mg 1 dose ) |
|
Side Effects |
Rash, pain, swelling, and a lump at the injection site; studies have shown that most side effects are mild and transient. | |
Vaccine Features |
Protective Effect: Reduces RSV-induced lower respiratory tract infections by 70-75% on day 150 after injection; reduces RSV-induced hospitalizations by 82.7% on day 180 after injection Protection Duration: Protection lasts for at least 5 months; antibodies last up to 1 year |
|
嬰兒出生至六個月尚未完成接種含百日咳成分的基礎疫苗,尤其出生後首2個月的空窗期,受百日咳感染程度最為嚴重,因而準媽媽更應在空窗期前作好防禦準備。
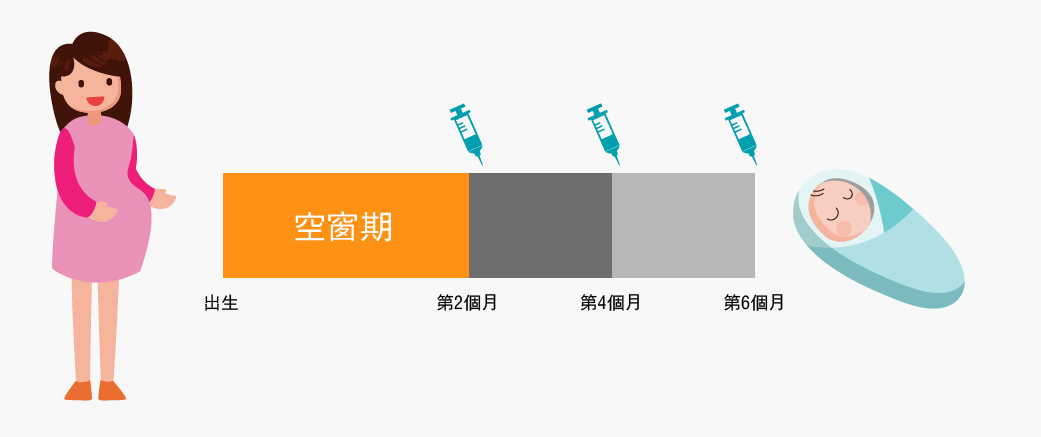
建議孕婦於懷孕第12至35週內接種一劑百日咳混合疫苗,讓疫苗在孕婦體內產生抗體,透過胎盤傳送給胎兒,讓嬰兒一出世便得到保護。

完成測試後,有機會獲免費骨質密度檢查 (DEXA) 乙次 或 骨質密度檢查優惠券乙張
閣下資料將會用作此推廣活動聯絡用途,如因資料有誤而未能聯絡閣下,本公司一概不負上任何責任。